It’s perfect time to use AI in healthcare.
AI systems are able to learn incomparably faster than human beings. This allows us to develop artificial experts tutored in numerous institutes in a short time.
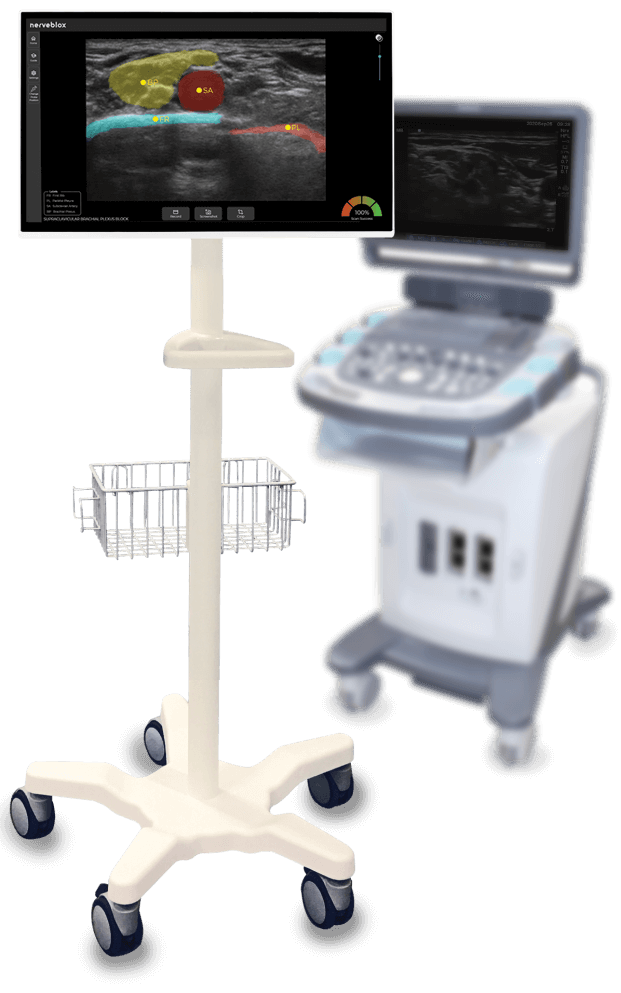
SmartAlpha precision is now mobile!
Alongside the re-certification of Nerveblox® under the new EU Medical Device Regulation (MDR), our new offering “Nerveblox for Android” is now also CE-marked for handheld use. It’s crafted to integrate with handheld ultrasounds to take AI guidance to new heights!
A huge shoutout to the SmartAlpha QA/RA team for their exceptional dedication and work!
https://www.linkedin.com/posts/smartalpha_smartalphaprecision-digitalhealth-ultrasound-activity-7115713108202991616-J6XJ?utm_source=share&utm_medium=member_desktop

AI systems are able to learn incomparably faster than human beings. This allows us to develop artificial experts tutored in numerous institutes in a short time.
Nerveblox is a CE marked medical device which indicates that it meets EU safety, health and environmental protection requirements.It is not available for sale in United States and this content does not constitute a clinical benefit nor a commercial offer.